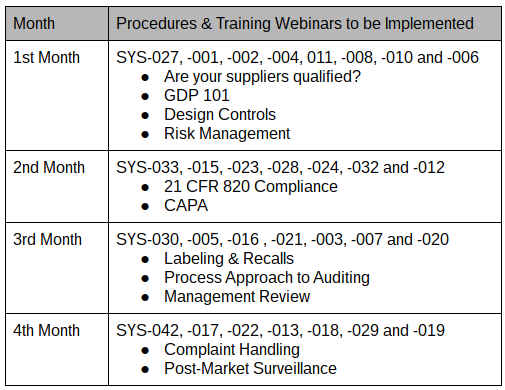
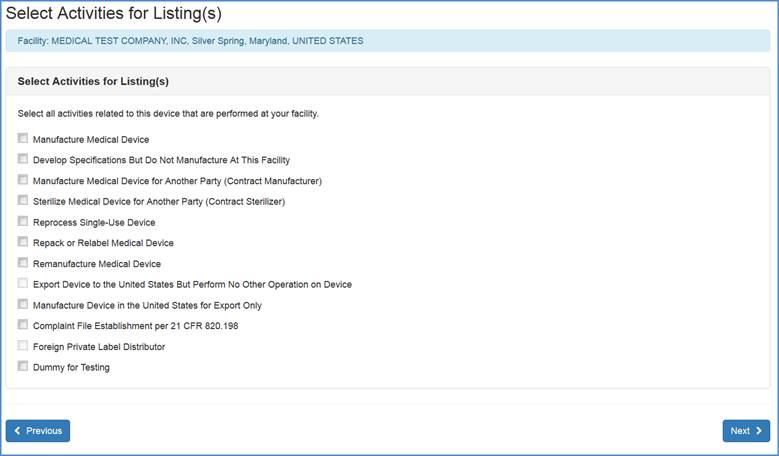
Medical Device Labeling Procedure
The following is a list of documents included.
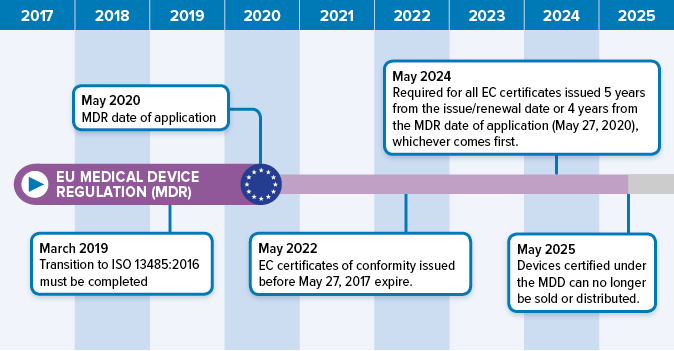
Medical device labeling procedure. 1 to assist manufacturers in their development and 2 to assist center reviewers in their review and evaluation of medical device patient labeling to help. In their notes they record the document number and revision of the procedure. These documents are updated for iso 13485 2016 and the new european regulations. Frm 033 a new eu mdr labeling requirements checklist.
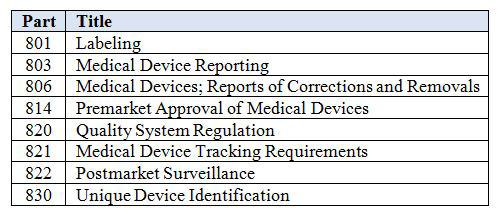
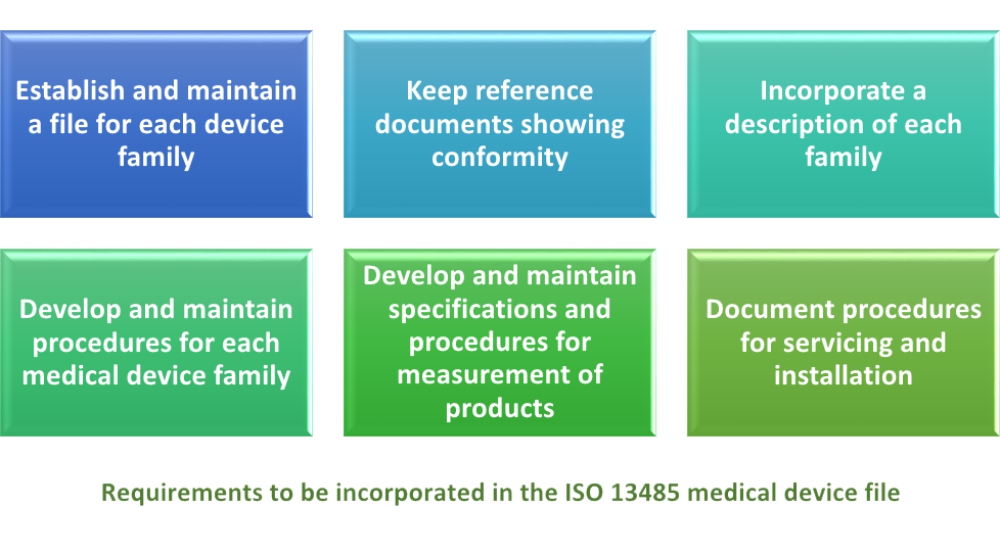
This guidance serves a dual purpose. Labeling checklist forms and labeling templates are included with the procedure. 2 2 other regulations exist with respect to medical device labeling e g. General device labeling 21 cfr part 801 use of symbols.
Sys 030 a labeling translation procedure. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations cfr. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations cfr. General device labeling 21 cfr part 801 use of symbols.
If your medical device company is planning to sell devices in the united states you will need to comply with the fda qsr for labeling and packaging control of medical devices found in 21 cfr part 820 120. 3 0 responsibility the president or a person delegated and assigned the task by the president. Most auditors and fda inspectors request a copy of a labeling procedure to verify compliance with the first requirement. 4 1 the essential requirements annex i of the mdd specify in paragraph 13 the minimum requirements.